Stannous Chloride Dihydrate is commonly used as an iron reducing agent (IRA) in the oil and gas industry. The material is capable of reducing ferric iron containing compounds to ferrous iron containing compounds in an acidic solution, such as one used for formation acidizing.
Stannous Chloride Dihydrate is commonly used as an iron reducing agent (IRA) in the oil and gas industry. The material is capable of reducing ferric iron containing compounds to ferrous iron containing compounds in an acidic solution, such as one used for formation acidizing.
Stannous chloride is also used in immersion tinning, as a catalyst, as a reducing agent, and a sensitizing agent for glass and plastics before plating. It also acts as an intermediate in tin chemical manufacture, as a soldering flux in acid electro-tin plating, and as an anti-oxidant and a corrosion inhibitor in canned carbonated soft drinks.
Request a Quote
We offer premium quality stannous chloride dihydrate and the most competitive pricing. Please contact us for a customized quotation.
Stannous Chloride Dihydrate Specifications
| CAS Number: | 10025-69-1 |
| Molecular Structure: | 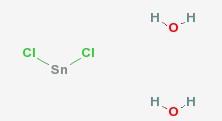 |
| Synonyms: | Stannouschloride dihydrate;Stannous dichloride dihydrate;Tin chloride dihydrate;Tindichloride dihydrate;
Tin(II) chloridedihydrate; |
| Molecular Formula: | Cl2H4O2Sn |
| Molecular Weight: | 225.63 |
| EINECS: | 231-868-0 |
| Density: | 2.71 |
| Melting Point: | 37-38 °C (dec.)(lit.) |
| Boiling Point: | 100 °C at 760 mmHg |
| Solubility: | 1187 g/L (20 °C) in water |
| Risk Codes: | 22-34 Details |
| Appearance: | colorless to white, odorless solid |
| Transport Information: | UN 1759/3260 |
| Material Safety Data Sheet |
Additional uses of Stannous Chloride Dihydrate
A solution of tin(II) chloride containing a little hydrochloric acid is used for the tin-plating of steel, in order to make tin cans. An electric potential is applied, and tin metal is formed at the cathode via electrolysis.
Tin(II) chloride is used as a mordant in textile dyeing because it gives brighter colours with some dyes e.g. cochineal. This mordant has also been used alone to increase the weight of silk.
It is used as a catalyst in the production of the plastic polylactic acid (PLA).
Tin(II) chloride also finds wide use as a reducing agent. This is seen in its use for silvering mirrors, where silver metal is deposited on the glass:
A related reduction was traditionally used as an analytical test for Hg2+(aq). For example, if SnCl2 is added dropwise into a solution of mercury(II) chloride, a white precipitate of mercury(I) chloride is first formed; as more SnCl2 is added this turns black as metallic mercury is formed. Stannous chloride can be used to test for the presence of gold compounds. SnCl2 turns bright purple in the presence of gold.
In organic chemistry, SnCl2 is mainly used in the Stephen reduction, whereby a nitrile is reduced (via an imidoyl chloride salt) to an imine which is easily hydrolysed to an aldehyde.[4]
The reaction usually works best with aromatic nitriles Aryl-CN. A related reaction (called the Sonn-Müller method) starts with an amide, which is treated with PCl5 to form the imidoyl chloride salt.
The Stephen reduction is less used today, because it has been mostly superseded by diisobutylaluminium hydride reduction.
Additionally, SnCl2 is used to selectively reduce aromatic nitro groups to anilines.[5] Aromatic nitro group reduction using SnCl2
SnCl2 also reduces quinones to hydroquinones.
Stannous chloride is also added as a food additive with E number E512 to some canned and bottled foods, where it serves as a color-retention agent and antioxidant.